SHANGHAI HONOR INDUSTRIAL CO.,LTD.
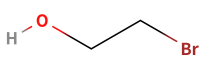
Bromoethanol
Bromoethanol
CAS NO.: 540-51-2
Content:98.00%
Application: Used as a building block for synthesizing pharmaceuticals, such as 2-piperidin-1-yl-ethanol via reaction with piperidine; also in selective reduction of nitroarenes using catalyst systems like PcFe(II)/NaBH₄. etc..
Description
Product Overview
2-Bromoethanol (also known as Ethylene Bromohydrin or Bromoethanol),, CAS No.: 540-51-2 , with the chemical formula C₂H₅BrO and a molecular weight of 124.96 g/mol. It is a colorless to pale yellow or dark brown hygroscopic liquid with a sweet, burning taste and relatively stable chemical properties, though it is toxic. This compound belongs to the class of halo alcohols and is primarily used in organic synthesis and industrial processes.
Properties
2-Bromoethanol exhibits the following key physical and chemical properties:
- Physical State: Liquid (at room temperature).
- Appearance: Colorless to pale yellow or dark brown liquid.
- Odor/Taste: Mild odor; sweet, burning taste.
- Melting Point: Not widely reported (typically low, around -50 °C based on similar compounds).
- Boiling Point: Approximately 150 °C (decomposes partially).
- Density: 1.59–1.60 g/mL (at 20 °C).
- Solubility: Miscible with water (forms an azeotrope); soluble in organic solvents like ethanol and ether.
- Stability: Relatively stable but hygroscopic; incompatible with strong oxidants, acids, and bases; may decompose upon heating.
- Toxicity: Toxic by ingestion, inhalation, and skin absorption; causes irritation to skin, eyes, and respiratory tract; classified as a hazardous substance (UN 1566, corrosive and toxic).
Applications
The primary applications of 2-bromoethanol include:
- Organic Synthesis Intermediate: Used as a building block for synthesizing pharmaceuticals, such as 2-piperidin-1-yl-ethanol via reaction with piperidine; also in selective reduction of nitroarenes using catalyst systems like PcFe(II)/NaBH₄.
- Solvent: Acts as a solvent and raw material in various organic reactions and chemical experiments.
- Industrial Processes: Employed in the production of surfactants, pharmaceuticals, and other fine chemicals; historically involved in ethylene oxide reactions with hydrobromic acid, leading to potential exposure risks in manufacturing.
- Other: Used in laboratory settings for bromoalkylation reactions and as a reagent in polymer chemistry.
Classification
The following table outlines the classification of 2-bromoethanol based on chemical properties, uses, and regulations:
| Classification Type | Specific Category | Description |
|---|---|---|
| Chemical Class | Halo Alcohol (Bromohydrin) | A β-bromo alcohol derivative of ethylene glycol, reactive in nucleophilic substitutions and prone to forming epoxides. |
| Usage Class | Organic Synthesis Reagent/Solvent | Primarily an intermediate for pharmaceutical and fine chemical production; limited commercial use due to toxicity. |
| Hazard Class | Toxic Liquid (UN 1566); Corrosive | Toxic (GHS: Acute Tox. 3, Skin Corr. 1B); irritant and corrosive; handle with protective equipment. |
| Regulatory Class | Hazardous Substance (REACH, TSCA) | Registered under EU REACH and US TSCA; subject to environmental and workplace exposure controls. |