SHANGHAI HONOR INDUSTRIAL CO.,LTD.
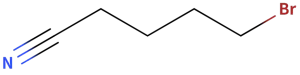
Bromovaleronitrile (CAS NO.: 5414-21-1)
Bromovaleronitrile (CAS NO.: 5414-21-1)
CAS NO.: 5414-21-1
Content:98.00%
Application: Used as a reactant to prepare key intermediates such as benzyl(methyl)amino)pentanenitrile for N-methylcadaverine synthesis; 1-cyanobutyl-3-alkylbenzimidazolium bromide salts by reaction with N-alkylbenzimidazoles; tridecanenitrile via Ni-catalyzed cross-coupling with dioctylzinc; and 5-bromo-pentanoic acid.
Description
Product Overview
5-Bromovaleronitrile (also known as 5-Bromopentanenitrile or 4-Bromobutyl Cyanide) CAS No.: 5414-21-1 , with the chemical formula C₅H₈BrN and a molecular weight of 162.03 g/mol. It is a clear colorless to light orange or yellow liquid. This compound belongs to the class of alkyl halides and nitriles and is primarily used in organic synthesis as a reactant and intermediate, though it is subject to safety regulations due to its toxic and irritant properties.
Properties
5-Bromovaleronitrile exhibits the following key physical and chemical properties:
- Physical State: Liquid (at room temperature).
- Appearance: Clear colorless to light orange or yellow liquid.
- Odor: Not specified.
- Melting Point: Not available.
- Boiling Point: 110-111 °C (at 12 mmHg).
- Density: 1.388 g/mL (at 25 °C).
- Solubility: Slightly soluble in water (4.1 g/L at 25 °C); miscible with organic solvents like ethanol and ether.
- Stability: Stable under normal conditions; store under inert atmosphere at 2-8 °C; incompatible with strong oxidizing agents.
- Toxicity: Moderately toxic; harmful if swallowed, in contact with skin, or inhaled; causes skin irritation, serious eye irritation, and respiratory irritation; no known carcinogenicity.
Applications
The primary applications of 5-bromovaleronitrile include:
- Organic Synthesis Intermediate: Used as a reactant to prepare key intermediates such as benzyl(methyl)amino)pentanenitrile for N-methylcadaverine synthesis; 1-cyanobutyl-3-alkylbenzimidazolium bromide salts by reaction with N-alkylbenzimidazoles; tridecanenitrile via Ni-catalyzed cross-coupling with dioctylzinc; and 5-bromo-pentanoic acid.
- Other: Employed as a building block in the production of pharmaceuticals, agrochemicals, and fine chemicals.
Classification
The following table outlines the classification of 5-bromovaleronitrile based on chemical properties, uses, and regulations:
| Classification Type | Specific Category | Description |
|---|---|---|
| Chemical Class | Alkyl Halide (Bromoalkane) / Nitrile | A brominated alkyl nitrile, reactive in nucleophilic substitution reactions. |
| Usage Class | Organic Synthesis Reagent/Intermediate | Primarily used as a reactant and intermediate in chemical synthesis. |
| Hazard Class | Toxic Liquid (UN 3276); Irritant | Toxic (Class 6.1, Packing Group III); harmful if swallowed (H302), in contact with skin (H312), or inhaled (H331); causes skin irritation (H315), eye irritation (H319), and respiratory irritation (H335); GHS07 Warning. |
| Regulatory Class | Controlled Chemical (TSCA) | Listed under US TSCA; WGK 3 in Germany; HS Code 29269090; subject to environmental and safety controls. |